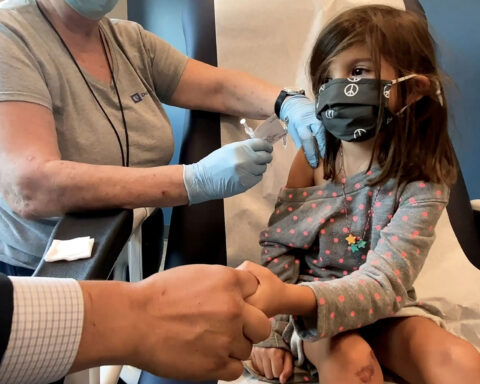
Vaccine advisers to the US Food and Drug Administration voted 17-0 with one abstention Tuesday to recommend emergency use authorization of Pfizer’s Covid-19 vaccine in children ages 5 to 11.
Members of the FDA’s Vaccines and Related Biological Products Advisory Committee agreed the benefits of vaccinating younger children appeared to outweigh the risks, but some members appeared troubled about voting to vaccinate a large population of younger children based on studies of a few thousand.
“It is reassuring to me that we are giving a lower dose,” said Dr. Paul Offit, who directs the Vaccine Education Center at Children’s Hospital of Philadelphia.
Pfizer has cut its vaccine to one-third of the adult dose for the children under 12 and said clinical trials showed this lower dose protected children well against symptomatic infection. The hope is it will cause fewer side-effects.
“We think that we have optimized immune response and minimized reactions,” Pfizer senior vice president William Gruber told the meeting.
One big issue was the theoretical risk of myocarditis — an inflammatory heart condition. It’s been seen in some people who got Pfizer and Moderna vaccines and is more common among young men, although it’s usually mild. Not enough young children were tested to show whether they’re also at risk.
“We’ve identified a lower dose which we expect is going to decrease the frequency of the rare side effect of myocarditos,” said Dr. Arnold Monto, chairman of the committee an a professor of epidemiology at the University of Michgan.
“I am just worried that if we say yes, then the states are going to mandate administration of this vaccine for children to go to school and I do not agree with that,” said Dr. Cody Meissner, a professor of pediatrics at Tufts University School of Medicine. “I think that would be an error at this time.”
But Dr. Peter Marks, who heads the FDA’s vaccine arm, the Center for Biologics Evaluation and Research, said that was unlikely.
“Just to reassure the committee, because we are taking an emergency use authorization rather than an approval, in general, although it’s possible that mandates could be put in place, I suppose, in general people have not done mandates with emergency use authorizations and there are certain governors who have already announced that they would not do a mandate until there was an approval as opposed to an emergency use authorization,” Marks said after the vote.
And Dr. Amanda Cohn of the US Centers for Disease Control and Prevention reminded the committee that children have died of Covid-19. According to CDC, more than 700 children 18 and under have died of Covid-19. “We don’t want children dying of Covid,” she said. “And we don’t want children in the ICU.”
The FDA had said that, under most of the scenarios it projected, the benefits of vaccinating younger children would outweigh any risks, and Pfizer said clinical trials showed the vaccine was more than 90% effective in preventing symptomatic infection in children.
The FDA will now take the committee’s vote under consideration and is likely to extend EUA to the vaccine for younger children in the coming days.
Then vaccine advisers to the US Centers for Disease Control and Prevention will meet next week, November 2-3, to discuss the decision and decide whether to recommend that US kids get the vaccine. The final word will lie with CDC Director Dr. Rochelle Walensky, and vaccination could begin next week if she gives the go-ahead.
The US federal government has a plan in place for delivering the smaller-sized vaccines to pediatricians’ offices, pharmacies and other venues across the country.
“The safety monitoring of this vaccine will continue. It has actually been quite intense,” Marks added.
Pfizer’s vaccine is authorized for youths aged 12-17, and a CNN analysis of CDC data shows about half of adolescents in this age group in the US are fully vaccinated.